State drug administration recently (CFDA) of the drug inspection report in 2016, the CFDA verification center for organizations to carry out the drug registration production on-site inspection, inspection of drug GMP certification, GMP inspection, flight check, imported drugs outside production site inspection, circulation of check and observation check a total of 434 items.
Among them, the flight check is a top priority - 2016 administration of receiving inspection task 45 times, complete and report the results of drug GMP flight check, 39 times in 2017 to carry out the other. Of the 39 times reported, 20 provinces, including Beijing, jiangsu and guangdong, have been reported.
In GMP flight tests, there are more problems with traditional Chinese medicine and biochemical drugs. The general administration has already dealt with the problems found in the inspection of the flights in accordance with the law, and the GMP certificates of 14 pharmaceutical companies have been recovered, including nine in TCM enterprises.
Chinese medicine companies found serious problems: in 2016, according to traditional Chinese medicine production enterprises, with 18 inspection group 66 flight check of 20 companies, including 12 companies do not conform to the requirements of the drug GMP, for 3 companies over the issues concerning the ShengJu processing, for 1 companies issued a warning letter, 1 companies related to production, conform to the requirements of the three companies.
The main problem is:
▍ proprietary Chinese medicine.
(1) changing the process problem without authorization is more prominent.
In order to reduce the cost of production, the phenomenon of pre-processing and extraction of the drug is an outstanding issue. This year for exploratory survey found that the problem to carry out the flight check, all is the enterprise will be part should be extracted in the prescription of Chinese herbal medicine is not according to the technical process to extract, but after feeding directly.
(2) the management of traditional Chinese medicine and Chinese medicine is chaotic.
Individual proprietary Chinese medicine production enterprises, in response to the pharmaceutical supervisory and administrative departments at all levels of the supervision and inspection, make warehouse material parameter and in-out warehouse records, according to the traditional Chinese medicinal materials production retracing the usage, dosage and on-demand fabricated material parameter.
(3) the purchase of Chinese medicinal herbs, Chinese medicine and Chinese medicine is not strictly examined, and the data reliability is problematic.
Traditional Chinese patent medicine production enterprise product variety, involving much more varieties of traditional Chinese medicine, Chinese medicine yinpian, due to the configuration of precision analysis instruments and QC personnel were not adapted to the scale of production, all can't promise to buy Chinese herbal medicine and Chinese medicine yinpian swarms, causes on the part of the batch of traditional Chinese medicine, Chinese medicine yinpian without a full inspection, or use a graph multi-purpose behavior to check.
▍ Chinese medicine yinpian
The contents of the products are highlighted, the problems of dyeing and weight gain have occurred, and the production records are of dubious validity.
The main questions include:
(1) the production record is not true;
(2) to be involved in the distribution and sale of foreign food and drink tablets;
(3) data reliability issues.
In 2017, it is a major disaster area for Chinese medicine and equipment
Since January 1, 2017, 33 medical enterprises have been released on the flight inspection report.
The most from the provincial distribution area, Beijing, reach four times, henan, Shanghai and hubei three times each, anhui, gansu, tianjin, guangdong and jiangsu each have two times.
From the inspection type, the sample was given priority to 13 times. At the same time, we can see that as people become more aware of their consumer rights, there are five of them.
From the type of enterprise involved, the medical instrument was the largest, up to 17 times, the Chinese traditional medicine and the Chinese medicine were nine and four times respectively.
Details of the following table:
The penalties for flying are increased, and traditional Chinese medicine is the worst-hit area
In the five months to 2017, 14 pharmaceutical companies have recovered from the GMP certificate, and 14 have been investigated.
Based on flight inspection report issued by sorting the CFDA finds: in order to guarantee the public medical health, to alert the illegal enterprise, the CFDA increased the penalties, year-to-date 14 GMP certificate of drug companies have been back, also have 14 drug firms are investigation, including GMP certificate at the same time be retrieved and initiate an investigation enterprise has 12.
Traditional Chinese medicine is a disaster area. The GMP certificate of 11 pharmaceutical companies has been recovered, while 11 pharmaceutical companies have been investigated, nine of which have been retracted GMP certificates and are being investigated.
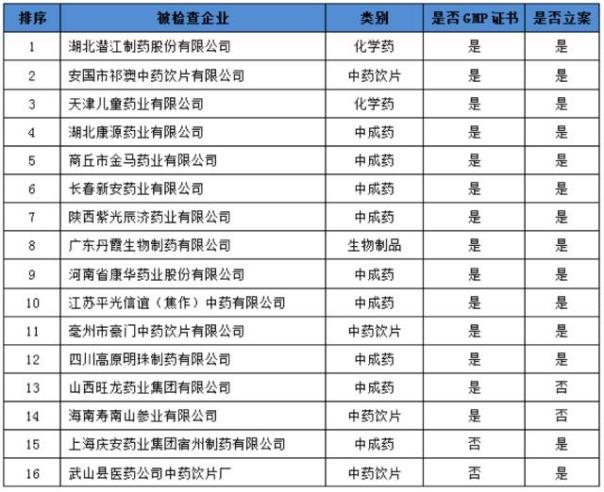
The list of companies that were retracted from the GMP certificate as well as the enterprise under investigation is listed as follows:
, 
Specific, relevant national regulators on fly detection problem of drug firms general treatment measures for recovery of GMP certificate, investigation, (production) rectification within a time limit, such as product recall. The relevant regulatory agencies are handling the following measures:
Attached: the main problem of 33 pharmaceutical companies examined
|
