| Micro-channel |
 |
|
|

|
|
|
| |
| Shanxi Province: 17 medicine to quit low price medicine, want to raise price? ! |
| |
| Author:中国铭铉 企划部 Release Time:2017-9-5 11:35:50 Number Browse:1286 |
| |
Two days before the September 5th medical bulletin, the list of new low-price drugs released by the provincial development and reform commission (NDRC) is over.
In recent days, shanxi provincial development and reform commission issued a notice to make a list of the list of low-priced drugs to be approved, and two days before the official deadline of September 7.
▍ low drug list changes
This time, on low drug list has 65 varieties, including isosorbide mononitrate, rifampicin and spray 49 kinds of western medicine, vitamin B complex, dong ling syrup, radix isatidis injection, liuwei dihuang soft capsule, fritillary bulb loquat syrup, etc. 17 kinds of proprietary Chinese medicine.
Finding cheap drug list there are 17 species, including pure tuberculin protein derivant, vitamin K1, such as seven kinds of western medicine, wuji baifeng pills, ageratum dropping pill for clearing and other 10 kinds of proprietary Chinese medicine, bezoar.
▍ transferred to bring up the low drug list
Shanxi Province from 2017 low-cost drugs dynamic adjustment work in early June, we have learned, to listed in the national and provincial drug list of species at a lower price, cancel the government formulated the highest retail price, the actual sales price calculation in medicine.
If the average daily cost of a low-price drug is not more than 3 yuan, and no more than 5 yuan for the Chinese medicine, the application report can be submitted.
Shanxi Province low - price drug to implement the voluntary declaration, the dynamic adjustment management mechanism.
Application for the inclusion of low-priced drugs:
(1) the average daily expense standard stipulated by the national price policy on drug prices;
(2) to accurately calculate the daily treatment quantity according to the medical instructions;
(3) comply with the price commitment to ensure the price of drugs is basically stable.
Conditions for applying for a low-priced medicine:
(1) the change in cost or usage may result in the average daily expense breaking the national low drug standards;
(2) failure to produce and supply due to insufficient raw material supply;
(3) the provincial price department of the locality has made a clear list of the low-priced drugs.
Shortage of cheap drugs and drugs are often of one wall lie between, the national development and reform commission issued the low-price drug list, allowing low-cost medicine prices in a reasonable scope, is to make cheap drugs to return to the market, guarantee the demand for treating patients.
▍ dual factor to low medicine as a shortage of medicine
The shortage of medicines is largely due to the fact that companies in the supply side have chosen to stop production. Is the rising cost, is the "low price strategy" in the bidding and medical insurance directory too "stable" drug prices cannot be improved synchronously with the policy environment, such as drug firms production enthusiasm is hit.
On the one hand, some low price drugs or close to the market price and cost price, profit is too low, or the market price can't cover the cost, the enterprise unprofitable, whether profits are too low or unprofitable, enterprise profit cannot meet the demand.
On the other hand, the cost of raw materials is increasing year by year. Take vitamin B1 for example. The price of one kilogram was 230 yuan, and now it is over 1800 yuan, sometimes even 2,000 yuan.
In addition to the policies of GMP standard certification and consistency evaluation, the upgrading of workshops and products has also contributed to the rising cost of drugs.
"Exiting the list of low-price medicines is likely to be due to the need for higher prices." The industry has been doing this to cypress blue.
The dynamic list of low-priced drugs is relatively quick to respond to drug prices, protecting the profit margins of pharmaceutical companies to a certain extent.
In June 2015, the national development and reform commission and other eight ministries jointly issued by the affairs of the commonly used low drug supply security work opinion "explicitly proposes that to change due to rising costs or usage, dosage, causing average daily cost breakthrough drug control standards at a lower price, to withdraw from the low-price drug list, redesign the highest retail guidance prices.
The attached:
Notice of the listing of the list of medicines listed in or issued by our province
Related pharmaceutical production and business units:
According to the national development and reform commission on notice to improve on the management of lower drug prices change (hair [2014] no. 2014) and "the Shanxi Province development and reform commission on or about application of low drug listing information notice" (jin change medicine is sent [2017] 2017), by commission business related information to declare for the audit, will now be listed in or out of the low price after checking drug list shall be published.
The public notice period is from September 1st to September 7th. If there is any objection, please return the written comments (the official seal) to the pharmaceutical price department of shanxi development and reform commission.
Lian: zhang fuping li kang
Contact number: 0351-3046652
Email: sxyyjg@sina.com
▍ attached: bring up the low-priced varieties of drug list
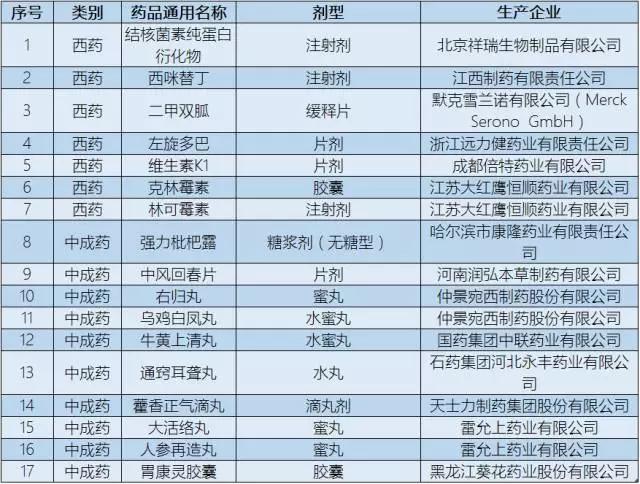
Attached: list of low - priced medicines
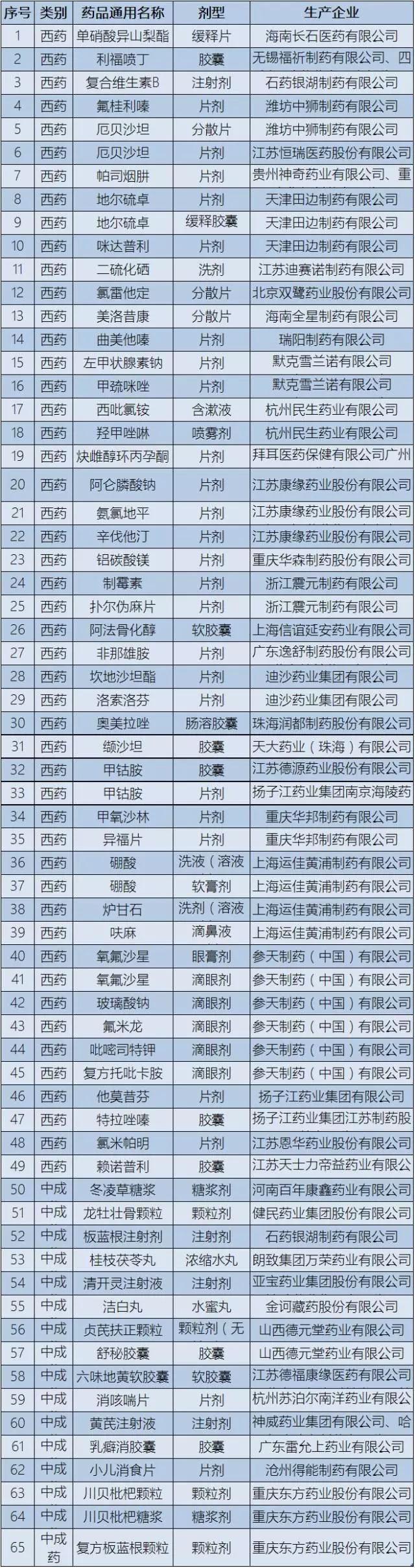
|
| |
Previous article:《Catalogue of medical devices》manual
Next article:《Catalogue of China's listed medicines》Ask for advice! 4 categories of products will be included
|
| |
|
|
