The drug purchase platform of yunnan province showed some companies voluntarily applying for a price reduction on September 13, 2009 (September 12).
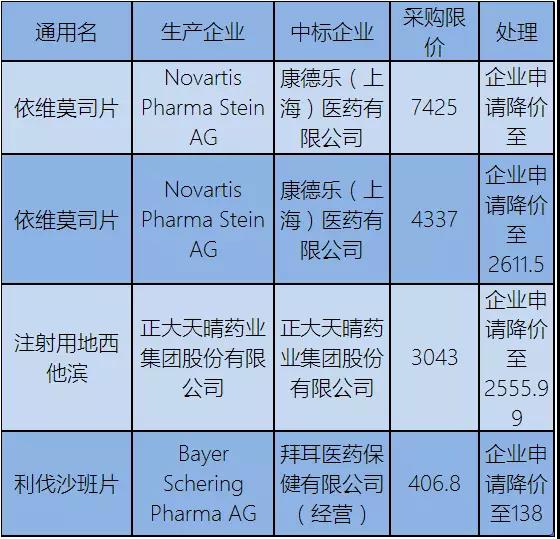
The apply for the price of drug firms have Kant, zhengda shine, bayer three, including in accordance with the dimension of therapy, sand injection land west he marina, cut class three drugs, including the felling shaaban drop as much as 66%.
▍ bayer, felling shaaban price cut
After liaoning and Shanghai, bayer's livastaban tablets (10 mg*5 tablets) also fell to 138 yuan in yunnan.
On August 31, according to the management of the Shanghai pharmaceutical intensive purchasing management office, the bid price for bayer's rivastabban tablet (10mg * 5) was lowered from 364.35 yuan to 138 yuan, a drop of 62%.
On August 21, a notice of price reduction was issued from the office of the leading group on pharmaceutical and medical supplies of medical supplies in liaoning province.
Bayer offered to reduce the price of rivastababan tablets (10mg * 5) to 138.00 yuan/box by the original price of RMB 327.92 yuan (10mg * 5).
Bayer's rivastabban cut its price in yunnan by as much as 66% so far.
▍ cut shaaban former glory
Rivastaban was the first Xa factor inhibitor to be developed by bayer pharmaceutical co., LTD., which was approved by the European Union in September 2008 and soon replaced warfarin.
The following year, baeyer introduced livastaban to China, and in 2009, the Chinese medical insurance directory was adjusted, and livarababan took the shuttle bus to the national health insurance directory.
According to the statistical sample of hospital in China, the market size of cutting shaaban from 2009 to 2016, 2.74 million yuan growth has been as high as 265 million yuan, in 2016, up 52.29% from a year earlier, the market share ranks third as heparin product's biggest threat.
▍ cut shaaban under siege
According to relevant data, so far, a total of 49 domestic enterprises in the cutting class generic declaration registration, including the weather is fine, jiangsu howson, step light ophthalmic pharmaceutical, koren pharmaceutical companies.
In addition to potential competition from domestic generics, other multinational drug companies also have products that pose a direct threat to barito.
BMS, for example, and Pfizer's apixaban. In 2013, the company entered the domestic market, after BMS management said that it had already exceeded the anticoagulant market prescription.
In addition to the alpakban, dabiega also entered the Chinese market and competed directly with rivastaban.
▍ medicare catalog readjustment, the market change
In early 2017, the national health insurance directory finally finished adjustment, the sand cut class of drugs in the original "limit" lower limb joint replacement surgery patients, on the basis of increased "limit of poor control of warfarin therapy or at high risk of bleeding in patients with nonvalvular atrial fibrillation", means that the sand cut class will have a broader market opportunities.
But after the good news, bayer's bad news followed. Two of the levaraban's rivals, dabiega and alperazam, are also included in the new edition, which ends with rivaroxaban's "health care period".
In foreign countries, the two products have gained momentum in recent years. In particular, the company's global sales in 2015 are $43.66 billion, and the global sales of the company are $1.86 billion.
In 2016, however, the company achieved 80 per cent growth, almost doubling its sales to $33.43 billion. It is hard to predict the outcome of the three products in the health care catalog.
In addition to the health care catalog, the patent cliff is another major crisis for rivastaban.
The patent period for rivastabban will expire in December 2020, with only three years left. In the face of the patent cliff, it is not unusual for drug companies to slash prices. In 2016, in the case of the patent cliff of the product wave, love teilon offered an 80% reduction in price, from 29,980 yuan to 3,996 yuan.
It is hard to say whether the price cuts will help the drug get out of the rut.
▍ attached: about enterprises apply for the price
|
