Last week, on September 15, China's first Chinese new drug for Chinese herbal medicine went to the public for approval, prompting the industry to rekindle the attention of the licensee (MAH) system. It has been nearly two years since the launch of the pilot scheme for drug listing licensee.
On September 6, zhejiang Kant pharmaceutical group co., LTD. Dan dragon oral liquid to declare approved by the state food and drug supervision bureau achieved new drug approval documents production, at the same time the company access to drug marketing authorisation holder, can produce the goods. This is the first time since the implementation of the pilot system of drug listing license holders in China. It also shows that our MAH system has entered the fast lane.
The first new drug to be approved
Danlong oral liquid is a new drug produced by zhejiang Kantian pharmaceutical co., LTD., which has been independently developed and developed. It will be monitored for 4 years for 6 kinds of new drugs. It has the function of qingping pingping and the relieving of phlegm, and is used for the treatment of TCM fever. Administration asked the product responsibility doctor and patient education should be strengthened, getting the product post-marketing adverse reaction monitoring, and completed according to the requirements of pharmaceutical management regulations Ⅳ phase of clinical trial, amplified sample, strengthen the safety and efficacy studies the goods, especially for asthma patient lung ventilation function before and after evaluation.
According to hu ziren, general manager of Kantian pharmaceutical co., it is mainly based on four points: first, the drug has independent intellectual property and is patented by the state. Second, the TCM theory of treating asthma has a major breakthrough. It is the first Chinese medicine to treat asthma with blood stasis. Third, safe and effective, modern preparation process production, quality and stability; Fourth, the MAH pilot has approved the first Chinese medicine new drug.
Pilot propulsion of MAH
From the draft in 2015 to 2016 in the formal solution, MAH system will be in Beijing, Shanghai, tianjin and other 10 provinces (municipalities) of authorisation from the production license, drug research and development institutions and researchers can also become a pharmaceutical marketing authorisation holder, greatly encouraged the development of medicine r&d staff enthusiasm, more made a great contribution to innovation medicine power in our country.
Drug marketing authorisation Holder (MarketingAuthorization Holder, MAH) system, usually refers to a drug drug technology research and development institutions, scientific research personnel, pharmaceutical production enterprises, such as the main body, through the proposed listing of drug licensing applications and drug license, approval documents and the drug quality mainly responsible throughout its life cycle of the system.
Policy timeline
Since the adoption of the draft MAH in 2015, the official document has been issued for about half a year, with the determination and strength of the country implementing MAH, and the timeline for the release of the policy is as follows:
, November 4, 2015, 12 session of the 17th meeting of the NPC standing committee decided to authorize the state council in Beijing, tianjin, hebei, Shanghai, jiangsu, zhejiang, fujian, shandong, guangdong, sichuan ten provinces and municipalities directly under the central government to carry out pilot, drug marketing authorisation holder system allows drug research and development institutions and researchers obtained drug approval number, take the corresponding responsibility for drug quality. Meeting to vote passed on the authorization of the state council in pilot implementation parts of drug marketing authorisation holder system and the decision of the relevant issues (draft), agreed to the state council to carry out the drug registration classification reform, improve the drug quality, promote the transformation and upgrading of China's pharmaceutical industry.
· on November 6, 2015, the CFDA published a pilot scheme for the approval of drug listing licensee (draft) to solicit public opinions.
· on June 6, 2016, the general office of the state council issued a pilot scheme for the system of the approval of drug listing licensee, and made a deployment of the pilot work on the system of the licensing of drug listed licenses. In Beijing, tianjin, hebei, Shanghai, jiangsu, zhejiang, fujian, shandong, guangdong, sichuan and other 10 provinces (cities) to carry out pilot work. The programme was implemented from the date of issuance to 4 November 2018. The drug production enterprises in the pilot administrative region shall be executed in accordance with the relevant provisions of the present scheme.
The time of arrival of each province and city
Basic 10 so far, in addition to the hebei province, the food and drug administration of pilot provinces (municipalities) have released their specific implementation plan, in hebei province in has also issued a "accelerate the notice of the pilot drug marketing authorisation holder system. In addition, the national CFDA level Shanghai pharmaceutical supervision bureau has a special section of "pilot program for drug listing license holders".
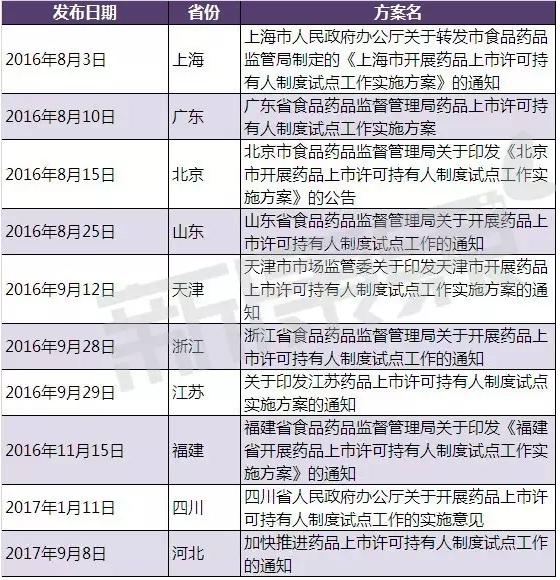
Source: the official website of the drug administration bureau of each pilot province
filing
On January 6, 2017, the CFDA counted and issued the declaration of pilot varieties of licensed drugs listed in the pilot provinces as of December 25, 2016. In terms of overall acceptance number, guangdong has the largest number, up to 47, with 39 applications for clinical trials. In terms of listing applications, Shanghai, jiangsu and zhejiang were equal to the first, with nine. In terms of supplementary application, Shanghai has the largest number of 15.
The list of application of the pilot varieties of drug listed licensee shall be declared
(up to 25 December 2016)
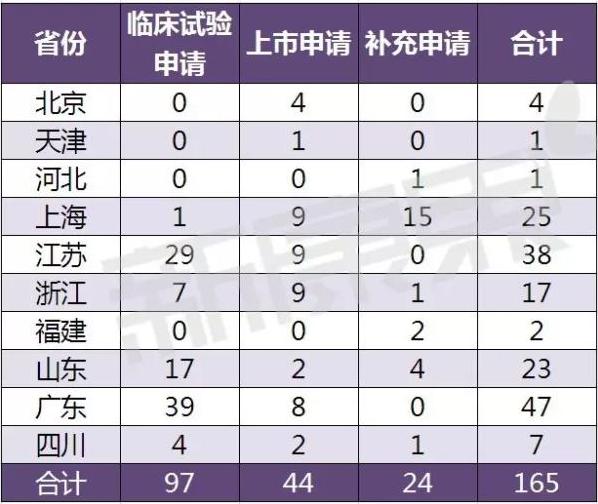
Remark: according to the acceptance number.
Approved by the situation
Has applied for drug marketing authorisation holder system reform pilot varieties of information is limited, from the CFDA website information division saw in shandong qilu pharmaceutical co., LTD., research and development of the treatment of anti-tumor drugs and tablet to become China's first drug marketing authorisation holder system pilot varieties. In addition, the application statistics released from various provinces and cities, there is now only Shanghai to r&d staff personal filing a MAH as the main body, the main application subject or production enterprises and research institutions.
On December 23, 2016, developed by shandong qilu pharmaceutical co., LTD. The treatment of antineoplastic drugs and tablet approved by the state food and drug supervision bureau, obtains the holder document number, become the first drug marketing authorisation holder system pilot varieties.
On March 27, 2017, zhejiang pharmaceutical co., LTD. Of malic acid nai, the effect of active pharmaceutical ingredients and its capsule approved by the state food and drug supervision and administration bureau made drug marketing authorisation holder document number, this is since the implement of drug marketing authorisation holder system pilot, benefit the first innovation of medicine.
, on July 21, 2017, the state food and drug supervision bureau approval suzhou to develop pharmaceutical co., LTD. Declare the original chemicals 1.1 amine drugs lu poke piece as drug marketing authorisation holder (MAH) system test varieties, and this is the first in jiangsu province has been approved as MAH system pilot varieties.
On September 6, 2017, zhejiang Kant pharmaceutical group co., LTD. Dan dragon oral liquid to declare approved by the state food and drug supervision bureau achieved new drug approval documents production, at the same time the company access to drug marketing authorisation holder. This is the first time since the implementation of the pilot system of drug listing license holders in China.
Two good directions
MAH is Europe, the United States, Japan and other developed countries and regions pharmaceutical practice in the field of drug regulation, and the drug management system in our country is still in step by step with international practice, therefore, listed on the pilot for three years accumulated a drug license after the management experience of the separation of the production license, fully implementing will become the direction of the trend of The Times. So, who will be the biggest beneficiary of MAH's implementation?
Taken together, the following two directions are positive:
Small and medium-sized drug research and development enterprises
Drug marketing authorisation holder system allows drug research and development institutions and researchers obtained drug approval number, changed the original registered only pharmaceutical producing enterprises can apply for pharmaceutical production, achieve the history of drug approval number, for the research and development of small and medium-sized enterprises in China is undoubtedly a major positive.
In the past, small and medium-sized research and development companies were unable to obtain the drug production approval of CFDA because they did not have a self-operated production plant, so that enterprises could only make a living by "selling batches". Authorisation and production permits the separation of liberated the innovative pharmaceutical enterprises, small and medium enterprises after the drugs listed permission, can entrust a CMO enterprises to production, and avoid the company holding the national and global advanced drug project has been in "selling" approval documents and to share the benefit from the drug after successful listed.
CMO company
CMO (Contract manufacturing Organization) enterprises generally rely on self-technology innovation and process improvement, have strong drug production and project management ability, and can undertake outsourcing services such as commissioning and production. The arrival of MAH will further promote the development and separation of pharmaceutical industry in China. Currently widespread overcapacity, Chinese medicine industry homogeneity competition more severe, the arrival of the MAH will encourage more have strong production capacity but enterprise turn for a CMO direction of research and development ability is weak, maximize their own production resources to undertake commissioned production outsourcing services, such as with resource integration, makes the production of core competitiveness.
conclusion
First approved the treatment pilot varieties and tablets from the pharmaceutical marketing authorisation holder system pilot program "less than six months after launch, and proprietary Chinese medicine every year to approved the first pilot varieties. Summarize approved several pilot varieties, can be found that the "first", "innovation" 2 words frequently, strong innovative drugs, market demand urgently two characteristics is more apparent, this to a certain extent, also represents the CFDA for MAH application criteria.
In addition, the pilot program stipulates that researchers can also be registered as a drug registrant, obtain a drug listing license and drug approval number, and may become a licensed drug licensee. But at present researchers still little personal as the main body of apply for MAH, investigate its reason, mainly lies in the pilot program required holding people on drug safety, efficacy and quality controllable negative overall responsibility, scientific research personnel to perform personal or difficult.
Though our propulsion of the drug marketing authorisation holder system pilot work issued a number of files, but there is still more shortage, declare quantity is less, it is difficult to for MAH pilot sums up experience and laws provide sufficient samples. Based on this, it can be inferred that the future expansion of MAH pilot scope may be the next key point of the pilot work.
|
