Medical network - on September 18, recently, liaoning, sichuan, fujian provinces of 17 pharmaceutical trading enterprises to "fly", among them, the four companies GSP certificate has been revoked, 12 were asked to make correction; Hainan, guangxi, xinjiang and other provinces and cities to clinic drugstore for centralized drug quality and safety regulation, integration of urban and rural areas and rural areas is the key, check several pharmacies and clinics was sent, details are as follows:
Liaoning: "flying inspection" 14 pharmaceutical wholesale enterprises
Recently, the food and drug administration of liaoning province announced the results of the flight inspection of 14 wholesale drug companies. The details are as follows:
|
企业名称
|
发现的主要问题
|
处理措施
|
|
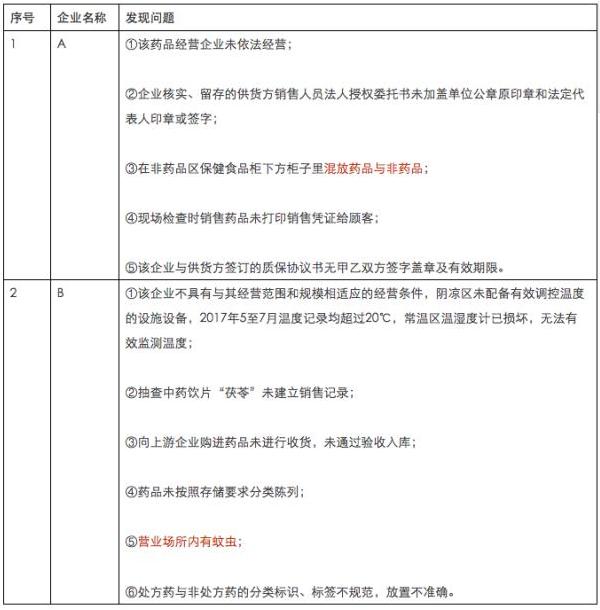
A
|
1.企业涉嫌虚构药品销售流向
2.企业部分经营数据未纳入计算机系统管理,经营行为无法追溯。
|
撤销GSP证书
|
|
B
|
1.企业2015年4月15日变更法定代表人、企业负责人,但未进行专项内审。
2.企业留存的上游供货商江西济华医药有限公司销售人员法人授权委托书未标明授权区域。
|
整改
|
|
C
|
1.企业2016年4月17日变更仓库地址,但未进行专项内审。
2.现场检查时中药饮片库温湿度均超标(湿度为82.7%、温度为25.4℃),保管员未进行有效调控。
|
整改
|
|
D
|
1.验收员金凤波兼职采购员。
2.部分药品不符合该品种的贮存要求,企业未拒收。
|
整改
|
|
E
|
1.16号温湿度监测终端人工干预超温至32℃,无声光报警及短信报警。
2.库房湿度超标,养护员未及时采取有效的调控措施。
|
根据对问题核查的结果作出最终处理决定。
|
|
F
|
相关人员未按药品温湿度监控系统管理规定进行及时调查、有效调控。温湿度探头(阴凉库1)声光报警后,企业人员未接收到报警短信。
|
整改
|
|
G
|
企业2017年7月10日销售红霉素肠溶胶囊(数量:300盒,批号:161250)随货同行单未加盖药品出库专用章。
|
整改
|
|
H
|
企业未及时更新个别供货单位印章样式。
|
整改
|
|
I
|
企业未及时更新个别供货单位印章样式。
|
整改
|
|
J
|
企业销售国家有专门管理要求的药品时,未对药品向下游企业销售情况进行有效管控。
|
整改
|
|
K
|
1.企业计算机系统不能对超出诊疗范围的下游客户进行销售管控。
2.企业未对购货单位的相关证明文件进行核实,不能保证药品销售流向真实、合法。
|
整改
|
|
L
|
1.企业运输冷链药品的运输记录随意涂改。
2.企业计算机系统不能自动生成药品运输记录。
3.企业未按照收货管理程序对购入药品的随货同行单与留存样式进行核对。
|
整改
|
|
M
|
企业经营中药饮片,但配备验收员李欣专业为药学专业,也无中药学专业技术职称。
|
整改
|
|
N
|
1.企业2017年1月至今计算机管理系统退货药品未形成销售退回验收记录。
2.企业阴凉库7号表模拟温度超标,无就地声光报警功能。
3.2016年1月16日、2016年4月4日分别入库20盒复方氨基酸注射液,计算机系统中进货采购人均为“无”,未自动生成采购人员。
|
整改
|
Hainan: sanya inspects the pharmacy clinic 64
Recently, sanya, according to the food and drug administration announced since July this year, the bureau of sanya urban-rural fringe and pharmacy clinics in rural areas for centralized drug quality and safety regulation, so far, a total of check pharmacies 39, clinic, 25, 51 issued a rectification notice, warning 18, 7 fine on the spot, case 4 cases, 1 cases adjudicated, FaMeiKuan totaling 7900 yuan.
Regulation: mainly through self-inspection and special inspection. Organization retail pharmacies and clinics for since January 1, 2016 drugs purchase channels, storage conditions and pharmaceutical care checking etc., on the problems existing in the rectification, form the comprehensive rectification report; Special inspection through adopts the method of divided area of responsibility to the people, giving play to the role of grass-roots accesses and coordinator, and more to the villages and towns market, pharmaceutical trading enterprises and medical organizations of supervision and inspection and dynamic monitoring.
Check key: antibiotics, Chinese medicine yinpian, precursor drugs, including special drugs compound preparations, biological products, human assisted reproductive technology commonly used drugs, illegal medical hairdressing drugs and devices, and treatment of sexual dysfunction, rheumatism arthritis pain, cervical spondylosis, lumbar disease, high blood pressure, diabetes and other chronic diseases commonly used drugs.
Main random drug purchase, acceptance record, monitoring the supply unit legal qualification, check its purchase channels is lawful, of dubious quality, source of drugs targeted of proper supervision, timely discover and investigate to produce fake and inferior drugs illegal.
Sichuan: panzhihua 1 pharmacy GSP certificate has been revoked
Recently, panzhihua. 1 food and drug administration for pharmacy flight check, found that there is a serious violation of the drug supply quality management norms "(GSP) behavior, decided to cancel the pharmacy drugs management quality management standard certification.

Guangxi: the quality and safety of pharmaceutical products carried out by wule food medicine
In order to thoroughly check the drug quality and safety hazards of drug stores and clinics in rural areas, regulate drug circulation order. Since July, the wule food and drug administration has organized and urged three pharmacies and 9 clinics in the area to conduct self-examination on the purchase, sale and use of drugs. Currently, it has recovered 11 copies of the self-report. According to various drug stores and clinics, in August, the drug control group organized the drug quality safety centralized operation. As of now, there are 3 pharmacies, 1 health clinic and 9 clinics.
Discovery of problems:
Some pharmacies have not been approved by licensed pharmacists to sell prescription drugs;
Do not ask for purchase invoices when purchasing drugs;
The drug is not strictly stored according to the storage requirements of the drug.
The result: the law enforcement personnel issued "notice of correction" on the spot, required the party to rectify and submit the rectification report and rectification photos within a time limit.
The next step will be to further expand the scope of the treatment to ensure the safety of people's drug use.
Xinjiang: aksu district carries out pharmacy drug quality safety centralized control
Food drug administration announced recently, aksu region, on September 11 - December 31, in the aksu area (county) city drugstores to carry out the centralized drug quality and safety regulation, to investigate and punish drug sales using link violation behavior, and ensure the safe and effective.
Key points:
Whether the pharmacist in the pharmacy is on duty;
Whether prescription drugs are sold by prescription;
According to the seasonal temperature change and whether to take necessary heat preservation or refrigeration measures;
The problems of drug and non-medicine, drug administration and drug administration, and the separation of drugs and general medicine.
Law enforcement officials require the drugstores to conscientiously do their own research, implement effective rectification measures, eliminate safety hazards and ensure drug market safety.
Fujian: the GSP certificate of 2 enterprises in xiamen was revoked
Recently, the fujian xiamen food, according to the food and drug administration announced to flight check of two pharmaceutical trading enterprises, two business a serious breach of drug quality management standard, decided to withdraw the drug supply quality management standard certification certificate.
|
