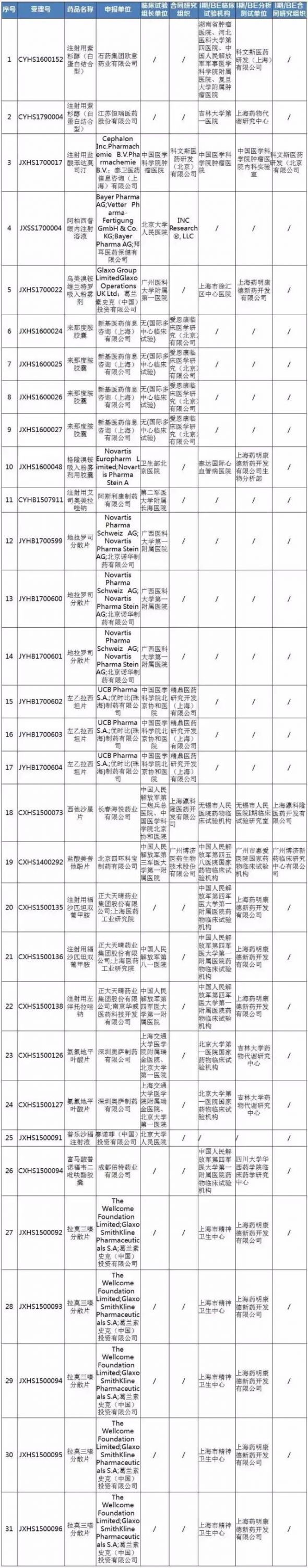
Medical network - on September 20th September 18, the CFDA food and drug audit inspection center website announcement of the drug clinical trial data field verification plan (14), according to the plan for injecting paclitaxel combined type (albumin) (accepted number: CYHS1600152) and other 31 drug clinical trial data inspection check varieties to carry out the on-site verification. The catalogue of the latest batch of clinical trial data for clinical trials of drugs is publicized, and the public notice period is 10 working days, which is September 18, 2017 solstice, September 29, 2017.
After the official period ends, the on-site inspection shall be conducted.
Drug clinical trial data site verification catalogue
|
