| Micro-channel |
 |
|
|

|
|
|
| |
| 30 billion anti-dementia drug market hospital, Top 10 |
| |
| Author:中国铭铉 企划部 Release Time:2017-9-22 10:03:50 Number Browse:2472 |
| |
In the 21st century, the world has entered an aging society, and the health of the elderly has become a social hotspot. In Chinese and foreign TV dramas, a number of shows depicting alzheimer's disease (AD) are now in front of the audience, leading to the thoughts on the life of elderly people with dementia later in life. September 21 is world senile dementia awareness day, caring for the health and tireless efforts to understand the senile dementia is an eternal topic.
Senile dementia is a progressive neurodegenerative disorder. Senile dementia is divided into three categories: alzheimer's disease, alzheimer's disease (AD), cerebrovascular disease, and mixed dementia. According to the study, AD may be related to genetic and environmental factors, and the disease is mainly manifested in the rapid attenuation of cognitive impairment and memory function, which severely affects the quality of life of the elderly. Alzheimer's is now synonymous with senile dementia.
According to the global alzheimer's disease report released by the international association of alzheimer's disease, there were 46.8 million AD patients worldwide in 2015 and 132 million by 2050. In China, the incidence rate of AD is 4% ~ 6%, and the number of people with dementia is about 6 million, and the number of people with dementia will double every 20 years. Senile dementia is a serious mental disorder, and active treatment and care has become a very urgent task.
Dementia has received widespread attention in recent years. Drug approved for AD treatment at home and abroad are mainly cholinesterase inhibitors and glutamate receptor antagonist, other neuroprotective drugs can be combined or adjuvant therapy, so as to promote the rapid development of senile dementia treatment at home and abroad market.
The new drug is not satisfactory
New drug research and development lines have been highly valued by drug companies, but few AD drugs have been approved by the U.S. food and drug administration for nearly a decade. So far, the FDA has only approved the glutamate receptor antagonist dollar, acetylcholinesterase inhibitor doneprazide, kabalatine, galanisin and he. The multinational pharmaceutical giant has been in the field of research in AD research. In 2016, the market of global AD original pharmaceutical market was just over $4 billion, showing a gradual decline year by year.
But as neural science and pharmaceutical markets both at home and abroad, under the attention of the whole society, the awareness, treatment of senile dementia has been improved, domestic AD drug market is another story.
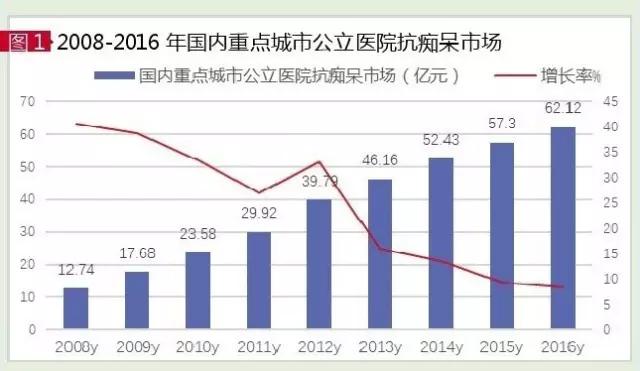
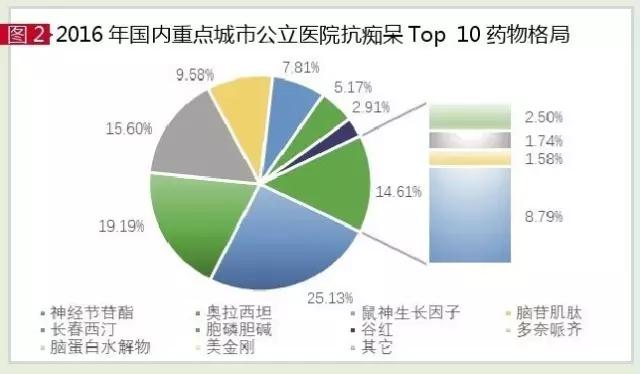
According to the data of the HDM system of CFDA, in 2016, the market of anti-aging dementia drugs in public hospitals in key cities in China was 6.212 billion yuan, up by 8.41% year on year. Clinical treatment and improved nearly 30 varieties of senile dementia drugs, TOP 10 drugs are single ganglia of sialic acid ester, olathe, mouse nerve growth factor, brain glycosides carnosine, vinpocetine, citicoline, GuGong injection, pp, brain protein hydrolysate and king kong. Under the influence of dementia caused by cerebrovascular disease in China, zhizhi auxiliary treatment drugs account for more than 80% of the total. In terms of hospitals and retail markets, hospitals are the mainstay of the anti-dementia drug market, accounting for more than 85 percent of the market, and the total market size in China has exceeded 30 billion yuan.
The dollar just got the upper hand
Dollars just is a kind of voltage dependence, moderate affinity noncompetitive NMDA receptor antagonist, was also the first used in the treatment of medium and severe alzheimer's drugs, in the 20th century 90 generation of development, is the main varieties of resistance to the AD market. In October 2003, the U.S. food and drug administration approved the U.S. dollar for forest company Namenda, which was officially launched in the United States in 2004.
The United States dollar has been combined with cholinesterase inhibitor doneprazide, kabalatine, galantamine and methyledrine to increase the therapeutic effect. In 2014 the FDA approved $Actavis and Adamas company just - donnelly, pp's fixed dose compound sustained-release preparation Namzaric new drug application, used for moderate to severe alzheimer's patients with dementia, the second quarter of 2015 by Actavis listed. Global dollar sales in 2015 were $1.747 billion, with the major brands being Namenda, lingbei/mak's Ebixa and the first three Memary.
In 2006, the dollar for Lundbeck in Denmark entered the Chinese market, and the goods were called Ebixa. On July 1, 2013, the CFDA approved the U.S. dollar raw materials for the federal pharmaceutical of zhuhai and its tablet and oral solution. The product is called yi times clear. On July 26, 2017, the CFDA approved the registration of usd new drug for the new pharmaceutical company in Beijing, becoming the second domestic manufacturer.
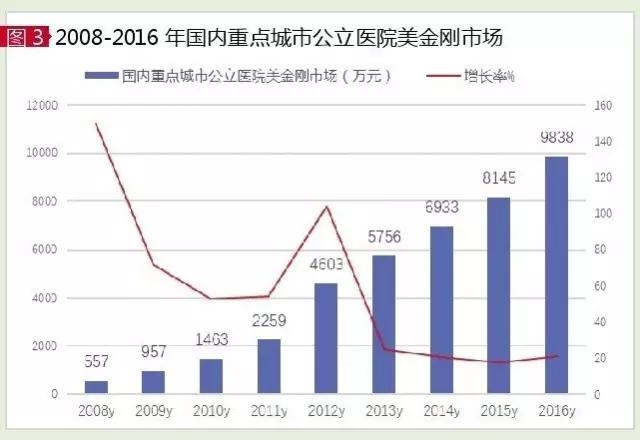
According to HDM system data, $2016 key cities for the domestic public hospital just drug market is 98.38 million yuan, year-on-year growth of 20.79% last year, is the domestic first-line drug resistance to senile dementia rapid growth of the market. Among them, the importation of drugs is easy to be accounted for 97.95% and the market share of 2.05% in the federation of zhuhai.
Doneperazi grew 19.35 percent
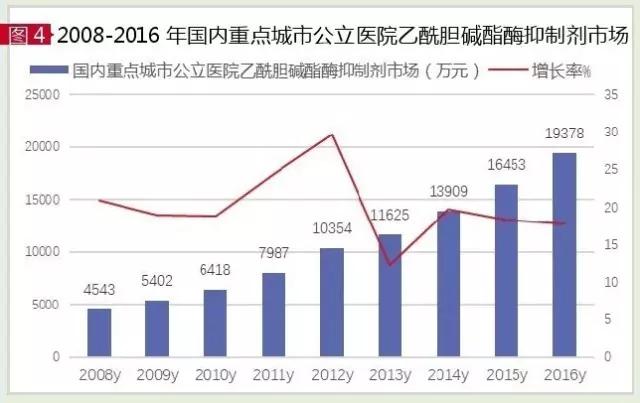
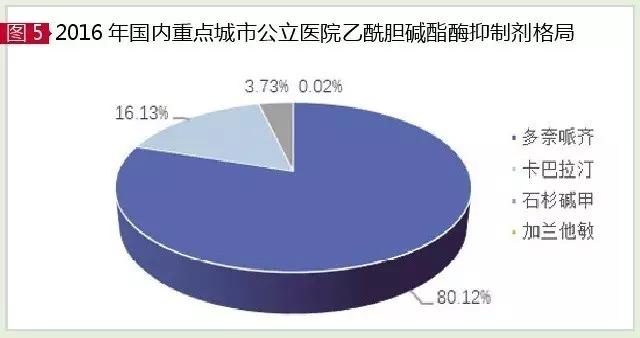
The current FDA approval of the acetylcholinesterase (AChE) inhibitor for AD is doneprazide, kabalatine, galanisin and he. According to the data of the HDM system, the market of AChE inhibitor in China's urban sample hospital in 2016 was 194 million yuan, up 17.78 percent year on year. Among them, donnepiazi, kabalatine and sugilina accounted for 99.98 percent of the total, and garland had a very small share, and he had been out of clinical practice.
The second generation of central acetylcholinesterase inhibitor doneprazide is a highly selective and reversible treatment of AD. On 25 November 1996, approved by the FDA, the joint development of the global market by Japan eisai and U.S. Pfizer was called Aricept. In October 1999, donnepiazi, listed in China as the name of the commodity, was the leading chemical agent against senile dementia. At present, CFDA has approved many domestic enterprises to produce donepezil, and the main dosage forms include tablets, capsules, dispersible tablets, and oral disintegrating tablets.
Donnelly, pp qi is version of health care in the directory 2017 varieties, sales of domestic manufacturers have eisai (China) pharmaceutical co., jiangsu howson pharmaceutical co., chongqing plant, pharmaceutical, xian haixin pharmaceutical, guizhou pharmaceutical st. Xavier hall, Yangtze river pharmaceutical group Shanghai heini pharmaceutical, shandong zibo new pharmaceutical and force was born in tianjin, the main brand name aricept, ares, spirit, noble, and strange, tusk, cover, bo sea and so on.
According to the data of HDM system, the sales volume of donepiazi of the domestic urban sample hospital in 2016 was 155 million yuan, up 19.35 percent from the previous year. The top three products are the safety of eisen, the poetry of jiangsu houson and the arris of the ark of shaanxi.
Kabalatin exclusive variety
Rivastigmine, fame and fortune, Ming, is approved by FDA for the treatment of senile dementia a pseudo not reversible acetylcholinesterase inhibitor, carbamate compounds, and physostigmine counterparts. The company's kabalatin is a domestically listed exclusive, known as Exelon. In 2016, Exelon's global sales were $444 million. In 2016, the sales volume of the domestic urban sample hospital was 312.6 million yuan, up 22.25% from the previous year, and the average growth rate was as high as 35.50% in 2012-2016.
Exelon selective inhibition of acetylcholinesterase of central nervous system and butyryl cholinesterase, especially has high selectivity to the hippocampus and cortex, increase the concentration of the synaptic cleft acetylcholine nerve cells, improve the clinical symptoms in patients with AD, cognitive function and mental symptoms, for each phase of dementia syndrome, with good compliance.
Olatasan is proud to be proud of the sitan class
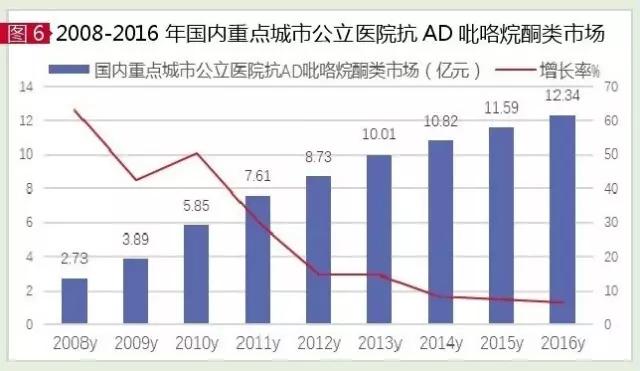
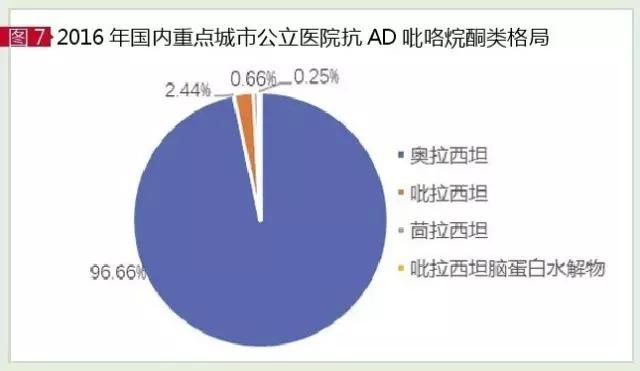
Olatasan and piracidan and anisalitan are the three commonly used brain metabolites that are commonly used in clinical practice, such as pyrrolidine derivatives, which were listed in China in the 1990s. According to the data of HDM system, the market of anti-ad pyrrolidine in sample hospitals in China in 2016 was 1.234 billion yuan, up 6.43% from the previous year.
Orazetam is the leading category of these drugs, accounting for 96.66 percent of the market. Olathe temple can promote the phosphoryl synthesis of choline and phosphoryl ethanol amine, increase the ATP/ADP ratio in the brain, can increase the synthesis of protein and nucleic acid in the brain, improve the patients with senile dementia and memory impairment of memory and learning capabilities.
Metacinonychia was inadequate
The natural medicine for the treatment of AD is a highly selective combination of cholinesterase competition and non-competitive. The drug from the snake an alkaloid extracted from Shi Sha, approved in China in 1994, its high lipid solubility, small molecules, easy through the blood brain barrier, after entering the central more distributed in the brain's frontal lobe, temporal lobe, hippocampus, in areas such as the increase in neural synaptic cleft acetylcholine content, can effectively treat memory loss and various types of dementia in the elderly.
The CFDA has approved the registration of 12 enterprises in China, including tablets, capsules and injections. Samples of 2016 domestic cities hospital huperzine a market only 7.23 million yuan, the TOP five manufacturers is the double benefit of fudan Shanghai after China ping (market accounted for 71.84%, the same below), Chen xin pharmaceutical industry (15.36%), henan too dragon pharmaceutical (8.38%), zhejiang yuan sangel pharmaceutical industry (3.35%), Beijing (1.07%).
|
| |
Previous article:Jiangxi yan dozen commercial bribery 90 days 11 medicine bribery by roll call!
Next article:CSO liberation, completely affect all the pharmaceutical companies!
|
| |
|
|
