Medical network - April 10
1. The drug registration continues to rise
According to m Intranet MED drug approval database statistics, overall in the first quarter of 2017 into the CDE monthly rise, application for registration of the drugs for new drug registration in March of 321 (according to the accepted number).
Figure 1:1 - March 17 years CDE in new drug registration application (according to accept no.)
(source: m Intranet MED drug approval database)
From the point of application type, nearly two months new drugs for new registration smoothly, the biggest increase the import application or not. It is important to note that on March 17, the CFDA official website of the imported drug registration management related matters about adjusting decision (draft) ", to encourage overseas unlisted boundaries of new drug approval in the synchronization of conducting clinical trials, import new drug applications don't have to wait for approval before they can submit at home, abroad on the imported drugs in China declare approval is undoubtedly a major positive.
Figure 1 - March 17 years I completely all types of new drug registration application situation (only new drugs, generic and imported)
(source: m Intranet MED drug approval database)
2. Domestic drug declare: March 17 class 1 new drug into the heart of the review
According to m Intranet MED drug approval database statistics, 2017 new domestic new drug application in March 63, involving 31 varieties, mainly chemical medicine 1 innovative drugs, including 17 varieties. ASK120067, SHR - 2042, morphine, ZL - 2306-6 - glucoside acid, hydrochloric acid, luo for like five varieties belong to special approval varieties.
Table 1:17 years march domestic new drugs to declare
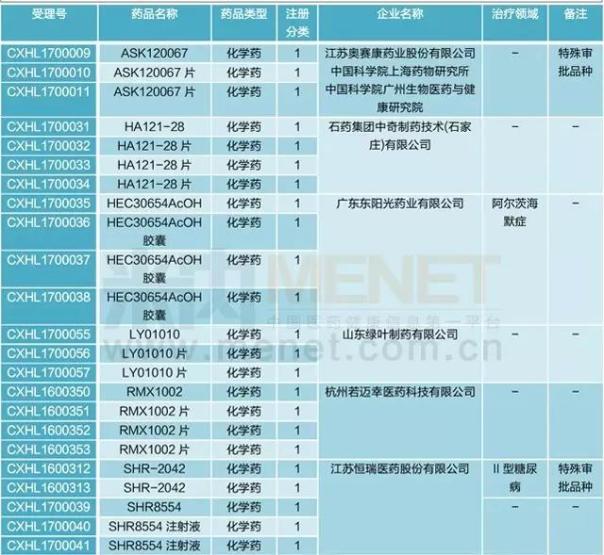
(source: m Intranet MED drug approval database)
March 3. Domestic generic declaration: generic declaration of chemical medicine
March domestic generic application of chemical medicine, according to the accepted number 51, mostly class 3 and class 4, belonging to 6 classes of generic application still has 20.
Table "March 17 years domestic generic declaration
(source: m Intranet MED drug approval database)
4. Import declaration: March 8 varieties in China to declare for the first time
CDE in March for new import registration 58, involving 32 varieties, of which chemical medicine 5.1 class to apply for the most. Ipatasertib, Lorlatinib, PT - 112 injection, Selexipag, cloth made zetham, Mr Lou amine soft capsule, without perindopril split indapamide amlodipine piece, MCS - 2 8 varieties such as the soft capsule for the first time at home.
Table 17 years his march import declaration
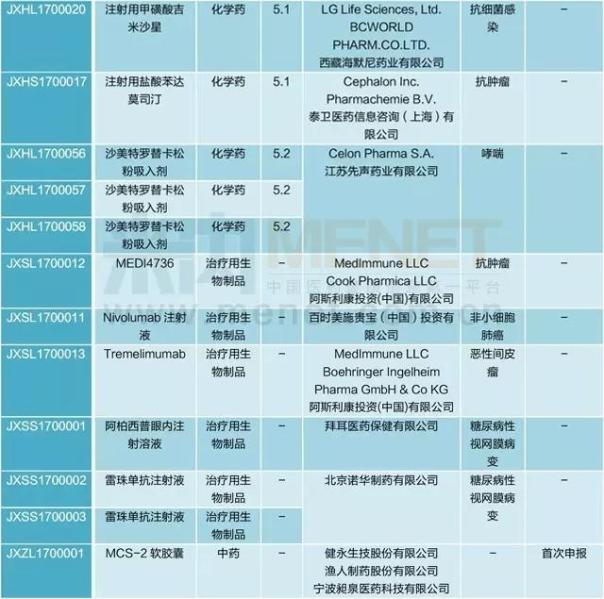
(source: m Intranet MED drug approval database)
5. Approval: four foreign varieties approved imports
According to m network statistics, the CFDA drug approval documents registered out 1124 in the first quarter. Influenced by public holidays, January and February overall low volumes, approval documents of the march of 681.
Image 1 - March 17 years CFDA drug registration certificate for distribution (according to accept no.)
(source: CFDA)
New drugs approved clinical situations
Table 4:20 on March 17 years some new approved clinical class 1 and class 1.1 new drug approval documents (by sending time statistics)
(source: m Intranet MED drug approval database)
Approved production/import situation
Table will March 17 parts approved production/imported varieties approval documents (by sending time statistics)
(source: CFDA, m Intranet MED drug approval database)
|
