Medical network on April 19 - Indonesia is the world's fourth most populous country, a population of about 257 million, 2015, per capita GDP of more than $3500. Though Indonesia the proportion is not high, population aging, the government introduced a series of reforms, including anti-corruption, cabinet travel spending cuts, investment license reform, cancel some of the administrative examination and approval, etc., and all staff should be brought into the national health insurance system, and have more citizens entering the middle class and the upper society, will bring new growth engine for the country's medical health industry development.
However, Indonesia's infrastructure is relatively backward, 2013 Indonesian infrastructure quality only ranked 82th, coupled with the merchandise and too dependent on natural resources, raw materials, relatively dispersed population, distribution of drugs is difficult. Therefore, the Indonesian market development faces many challenges.
However, for those who dare to face challenges and hope the cooperation with local enterprises, long-term returns is still huge.
Double-digit growth in the pharmaceutical industry
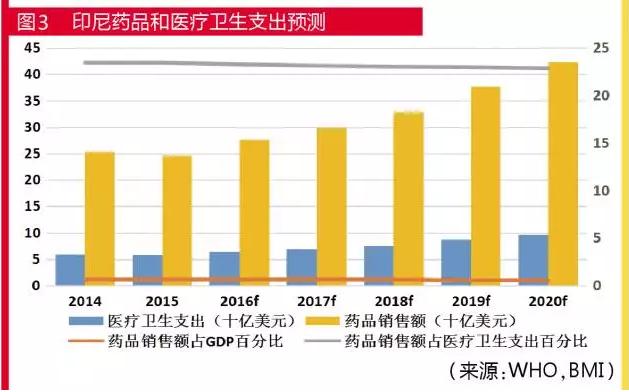
Indonesian pharmaceutical industry has developed rapidly in recent years has maintained double-digit growth. Pharmaceutical sales in 2015, Indonesia 2015 rupiah ($5.8 billion), accounting for medical and health care ($24.7 billion), 23.5% of the total in 2016 is expected to reach 2016 rupiah ($6.4 billion), 10.2% year-on-year rate of increase in local currency terms, in dollar terms year-on-year growth of 10.9%.
Imported drugs through local distributors
More than 200 across Indonesia about pharmaceutical producing enterprises, among them 168 domestic enterprises, 38 foreign companies, 95% of the Indonesian market by local companies, but the drug sales of domestic enterprises only accounts for less than 75% of the entire market share.
Nearly 80% of the pharmaceutical factory is located in Jakarta, the capital of Indonesia and Java area, such as tablets, syrup, ointment, capsule production.
Kimia Farma is Indonesia's largest state-owned enterprises, has a pivotal position in Indonesia market, in which the government controlled about 90%. Kimia Farma has more than 600 pharmacies, more than 50 clinic and laboratory, subordinate distribution company has more than 40 warehouse, accounted for 19% of Indonesia the pharmaceutical distribution market scale.
In addition, including sanofi, bayer, bristol-myers squibb, schering-plough multinationals such as operates production plants in Indonesia. In September 2016, sanofi dengue vaccine production for Indonesia food and drug administration for approval, indicates the enterprise's business in Indonesia will be further developed. According to the WHO, after Brazil, Indonesia is the world's second largest dengue disease. While other multinational companies through the way of exports, from wholesalers in the local distribution of drugs.
OTC products demand
Indonesia local companies dominated the generics and OTC drug market, OTC products accounted for 40% of the Indonesian pharmaceutical market share, while in other countries that share is about 10% ~ 10%, the market sales of OTC products 75% controlled by local enterprises, foreign enterprises accounted for 25%.
Lack of health insurance to pay high medical costs and prescription drugs, has low cost and rapid growth of over-the-counter (OTC) market, and the increase of infectious disease further stimulate the demand for preventive OTC drugs, pain relievers and digestive drugs of high demand for the largest contribution to the OTC products market share, followed by vitamins and dietary supplements to prevent such OTC drugs, cold and fever medication use is also showed rapid growth trend.
Traditional herbal high recognition
Indonesia for traditional herbal recognition is higher, there are about 1243 domestic traditional herbal medicine manufacturers, of which 10% larger, the remaining 90% of producers is the size of the small and medium-sized, mainly located in the Middle East Java. Nonetheless, only a handful of traditional herbal medicine company can satisfy the Indonesian food and drug administration regulation, it also forced some cannot enhance the level of production started enterprise merger, acquisition and reorganization. According to Indonesia's herbal and traditional medicine enterprises association statistics, Indonesia's traditional herbal medicine sales began to decline from 2009, the main reason for the massive influx of Chinese production of traditional Chinese medicine in Indonesia market.
Nyonya Meneer and SidoMuncul is Indonesia's largest producer of two traditional herbal medicines, the two accounts for nearly 30% of traditional herbal medicine market share, they produce the high quality of proprietary Chinese medicine, the health care beverage and dietary supplements, and the products are exported to eastern Europe, Russia, Malaysia, Singapore, the Philippines, Taiwan, Saudi Arabia, the United States and New Zealand, etc.
Active pharmaceutical ingredients, equipment import dependency
Indonesia for more than 1300 kinds of active pharmaceutical ingredients, 90% dependent on imports, a major importer for China and India. Indonesia's special geographical environment, the domestic infrastructure is not perfect, laws and regulations to carry out the insufficient, lack of funds and the government's leading API production investment cycle is long, etc., which limits the development of active pharmaceutical ingredients.
Medical devices and home care equipment must be registered in Indonesia food and drug administration, and food and drug administration shall have the right to cancel the registration. Local production of medical devices must submit an application for registration by local enterprises, import medical instruments must be cases, authorized by the foreign registration from local importers.
Indonesian domestic production of medical equipment accounted for only 15% of market share, and mainly for basic goods such as crutches and a wheelchair and one-time consumables. Most products rely on imports, the main source of imports to the United States, Germany and Japan, Johnson & Johnson, GE and BD company in Indonesia market leadership, including general electric (GE) has become a ct scanner in Indonesia, one of the biggest suppliers of philips in Indonesia cities set up sales outlets, to become mobile midwifery monitor market leader.
The huge market space
Existing medical level is weak
In 2015, Indonesian health care spending as a proportion of GDP is about 2.9%, the per capita health spending $299 a year. Men's life expectancy of 67 years of age, and the women's 71 years old, at the same time infant and maternal mortality has fallen dramatically.
Indonesia, about 1.55 million people die each year, noncommunicable disease mortality rates as high as 71%, including cardiovascular disease ranked the first, accounted for 37%, infectious diseases, perinatal death and deficiency disease (22%), cancer (13%), diabetes (6%), chronic diseases (5%), injury deaths accounted for 7%, other diseases (10%). 30 ~ 70 years old in the cause of death, cardiovascular disease, cancer, diabetes and chronic diseases (23%), cervical cancer is relatively common, lung cancer incidence is increasing.
Western diet and lifestyle is becoming more and more popular in Indonesia, lead to a surge of diabetes, also indirectly cause other lifestyle-related disease. In 2004, the number of diabetes for 35000 people, and by 2012, the death toll caused by diabetes mellitus has more than 100000 people. According to WHO statistics, in 2012, the number of strokes in Indonesia to 328500, ischemic heart disease incidence of 138400 people, a combined accounted for 30.1% of the deaths. In addition, the lower respiratory tract infection, liver cirrhosis, chronic obstructive pulmonary disease, hypertensive heart disease, kidney disease incidence in more than 40000 people a year.
Local infectious disease epidemic
In Indonesia, the high prevalence of infectious diseases, and every day, about 1500 children died of tuberculosis, malaria, pneumonia, 45% of the population affected by malaria. , according to the WHO in Indonesia, 30 people in every 100000 people with HIV/AIDS and tuberculosis, has 10 people died of AIDS and tuberculosis. In 2015, Indonesia about 26000 people died of AIDS and tuberculosis. , Indonesia, a total of 330729 tuberculosis cases reported, including new and relapse of 328895 cases, in these patients, only 32% of the people will be able to get treatment. In these cases, 11% of people living with HIV, 10% of people have been diagnosed with HIV positive, but only 21% of HIV positive patients were able to get treatment. Indonesia malaria incidence is high, as early as in 2013, confirmed cases of malaria has reached 250000 people, including falciparum malaria (57%), vivax accounted for 43%.
In addition, due to the medical and health conditions in some regions is relatively low, Indonesia nearly half of women choose to have children at home, in the maternal mortality rate is higher. And causes of death in children under the age of five in the survey found that premature deaths (19%), acute respiratory infection (16%), congenital malformations (11%), birth asphyxia (11%), injury (7%), neonatal sepsis (6%), diarrhea (6%), 5% of measles, malaria, accounted for 2%, HIV/AIDS (1%).
Medical and health new power
In Indonesia, only a few people have health insurance, most people rely on the public health system, serious shortage of health care equipment, free health care cover only a small portion of the population, mainly government employees. In some rural areas, some communities can implement some self relief program, the participants pay a fee, to establish a joint fund, can be in a local hospital outpatient service centers and clinics for medical services and medicine.
In 2014, Joko Widodo after the President's first instruction is, to 2018 years ago to double healthcare spending. Under this directive, the Indonesian government will spend most of the funds used to improve infrastructure such as hospitals, health care and have a positive impact on the pharmaceutical market.
As the government to improve medical and health system, increased drug accessibility become the key factor, Indonesia in 2014 universal health coverage JKN plans are put forward. The plan period for 5 years, covering population will increase from $2014 in 122 million to 2019 in 257.5 million. According to the JKN plan, expected 19% of medical and health care spending will be used for medicine. Indonesia is now in the follow up plan to adjust JKN essential medicines list, is expected to adjust after the essential medicines list on 92% of the drugs for low-cost generic drugs.
On June 8, 2016, Joko Widodo signed in June 2016 call to arms, accelerate the development of pharmaceutical and medical equipment industry. The ministry of health is responsible for compiling the specific implementation plan development self-sufficiency of the pharmaceutical industry. At the same time, to simplify the process of examination and approval of pharmaceutical factory. And social security bureau to promote pay local hospitals and health clinics for reimbursement ability. The ministry of finance to develop incentives to attract pharmaceutical industry investment, investment coordination bureau to provide services, formulate specific new policies to promote investment in pharmaceutical field. According to statistics, compared with 2006, 2016, the government in health care industry investment will increase by 13.9%.
The new patent law for generics
Indonesia on July 28, 2016 of the patent law amendment is approved and will take effect on August 28. Amendment for drugs on copyright exception and clear the compulsory license, drug patent tort exceptional circumstances include: without the permission of the patentee import patented in Indonesia and in other countries legitimate sales of the drug; For permission and listed after the expiration of the patent in the patent within 5 years prior to the expiration of the valid period of production of patented drugs in Indonesia.
According to the relevant institutions, pharmaceutical import exception is aimed at helping to ensure the reasonable drug pricing and increase the drug supply. If prove a drug in Indonesia for same product price is too high, compared with the international market, which can be used. Indonesia will Bolar exception from 2 years to 5 years, makes the generics firms have a longer time for research and development before the expiration of the patent registration, to speed up the generics market, the process to the public as soon as possible for quality and cheap drugs will become more prominent role.
In spite of this, the food and drug administration in Indonesia (NADFC/BPOM) laws and regulations has not been before changing the generic drug manufacturers also can't use the clause in practice.
In addition, the amendment also explicitly banned to second second medical use or use existing patent protected. If a developing country and least developed countries require the use of a patented drugs in Indonesia to treat an endemic diseases, the amendment allows the compulsory license may be granted the drug can be produced in Indonesia and then exported to the countries, if the drug is not produced in Indonesia, you can to the country's government awarded the compulsory license, allowing it to an endemic import purchasing a drug treatment, which relies heavily on the government's discretion.
China and India trade ten years increased nearly fourfold
China Indonesia pharmaceutical trade development is rapid, the 10 years from 2006 to 2015, the bilateral imports and exports year-on-year growth of 27.7% on average, to promote bilateral pharmaceutical trade rose from $2006 in 310 million to $2015 in 1.17 billion. In addition to the affected by the global financial crisis in 2009 has dropped sharply in the medical trade growth in China and India, the rest of the year basically achieved a relatively rapid growth.
In 2016 the import and export data
1 ~ 11 months in 2016, our country medicine and health products import and export of $93.397 billion, up 1.06% from a year earlier, while Chinese medicine trade between $1.074 billion - Indonesia, edged up 0.87% year-on-year, the basic flat with last year, mainly involved in TCM products in our country, especially for volatile oils such as eucalyptus oil extract caused by a sharp drop in exports to Indonesia.
Exports, Indonesia is my pharmaceutical products for the association of south-east Asian nations (asean), the second largest export market. In 2015, our country export of pharmaceutical products for Indonesia $998 million, up 3.29% from a year earlier.
Main export still to western medicine class, especially chemical pharmaceuticals. 1 ~ 11, 2016, our country western medicine products exports to Indonesia $648 million, including chemical API about $464 million, up 12.99% from a year earlier, accounting for 61.85% of the total amount of China's exports to Indonesia pharmaceutical, chemical and biochemical medicine exports have also maintained a relatively rapid growth, growth of 4.5% and 4.5% respectively.
Medical device products in recent years the basic showed more than 20% of the growth trend, but since last year, medical apparatus and instruments for the Indonesian export growth slowing, 2016 1 ~ 11 month appear even negative growth, mainly due to the hospital with a washbasin and massage health instruments, such as caused by a sharp drop in exports.
Indonesia because of the Chinese is more, have a tradition of herbal medicine, the state also encourages the use of herbs, and traditional Chinese medicine in China for Indonesia rapid growth of exports has remained relatively stable. But as a result of a few years ago for some plant extracts plant-based product inventory is more, 1 ~ 11 months in 2016, plant extracts and Chinese herbal medicine yinpian appeared a larger degree of decline in exports to Indonesia, exports fell 44% % and 11%, respectively.
Imports, China's pharmaceutical products imported from Indonesia is less, mainly focused on health and some medicinal plants, medicinal herbs and essential oils such as bird's nest, frankincense were the blood products.
Opportunity and challenge of China
From the demand and the policy level, Indonesia is a worthy of pharmaceutical companies to enter China market. As Indonesia has great potential for development of medical market, pharmaceutical industry base is relatively weak, high import dependency, API is extremely strong demand in China. At the same time, the Indonesian government intends to develop traditional herbal medicine, local residents and the use of Chinese traditional medicine to have the strong interest of Chinese indonesians, this foray into Indonesia has created favorable conditions for Chinese medicine.
Enter this market, however, still faces a big challenge. First, China's exports to Indonesia still is given priority to with active pharmaceutical ingredients, the preparation of high added value products in Indonesia import proportion is still low, at present the imported drugs procurement every year in Indonesia, China accounts for less than 5%; Second, although Indonesia need medical equipment imports 90%, but the importer comes mainly from the United States, Europe, Japan and other developed countries, China's exports of medical equipment in Indonesia are still focused on areas of low end products such as disposable supplies, health supplies; Third, there are many islands in Indonesia, transportation cost is big, does not allow foreign enterprise directly in the local distribution, drug and big pharmaceutical and medical equipment distributors for supplier selection more picky, and small distributors integrity aspects have a higher risk, increase the into difficulty. |
