Medical network, June 9, with the wide use of antimicrobial agents, dysbacteriosis in the human body, lead to drug-resistant strains, but also make the organization of deep fungal infection rates rise.
On the other hand, with the increase in the number of patients with human immunodeficiency disease, human organ transplantation, bone marrow transplant, minimally invasive interventional diagnosis and treatment and the use of antineoplastic chemotherapy drugs, and had received peritoneal dialysis population growth, the risk of systemic fungal infection has surfaced, incidence of fungal infection at home and abroad present a rising trend year by year, objectively promotes the rapid growth of antifungal drug market.
General situation of
It exceeded 22 billion yuan
Fungal infections are often seen as minor ailments such as athlete's foot, tinea and dandruff, which are only superficial fungal infections. In fact, serious fungal infections have been a major threat to humans, and the global depth of the fungal infection is worsening. Data show that can cause disease of about 600 kinds of fungi, one of the most common is candida, music bacterium, cryptococcosis and pneumocystis, accounts for about 70% of all fungal infection. As many as 1.5 million people die from fungus infections worldwide each year, challenging the traditional market for antifungal drugs.
According to m Intranet "China city public hospital chemical medicine terminal monitoring analysis system" (hereinafter referred to as the HDM system) data, in 2016, domestic major cities public hospital antifungal medicine market scale has reached 2.075 billion yuan, in 2010 compared to the same public hospital antifungal drugs 1.106 billion yuan scale increased by 88%. The total body was 90.03% with antifungal drug and 5.41% for gynecological urinary system and 4.56% for external antifungal drugs.
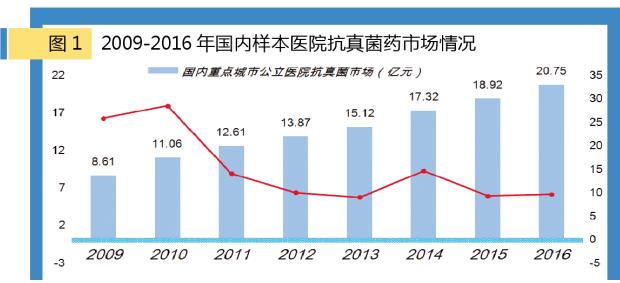
According to the market analysis of antifungal infection terminal, the whole body has an absolute share in the market of grade hospitals with antifungal drugs. As an important component of OTC products, the gynecological urinary system antifungal and antifungal drug use is a significant part of the OTC category, which accounts for a small share of the market in grade hospitals. Domestic urban public hospitals, public hospitals at the county level, the urban community health care, in towns and townships, entity drug stores, shop three terminal 6 big market of the scale of antifungal drugs have reached 22 billion yuan.
The TOP 5 variety accounted for 88.32%
The 2017 edition of the national health care catalog, which carries a total of 9 varieties of antifungal drugs, USES 20 varieties of anti-fungal and urinary antifungal drugs.
In 2016, the domestic main city public hospital antifungal drug market there are 43 varieties, including TOP 10 drugs are voriconazole, mooring Finn net card, fluconazole, mika Finn net, itraconazole, than naphthalene Finn, posaconazole, amphotericin B, biphenyl benzyl, naphthalene for Finn ketone health zun. The five leading drugs in the market accounted for 88.32% of the market for antifungal drugs. The net growth of voliconazole, carapine, fluoroconazole, and micacin. Itriconazole and amphoterycin B showed a decline.
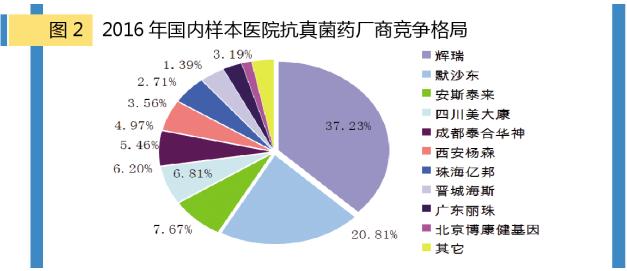
In 2016, the market for anti-fungal drugs in public hospitals in China's major cities is still dominated by foreign companies. The TOP 5 manufacturers are Pfizer, silent shandong, anstam, sichuan meidkang and chengdu tai-hua god, accounting for more than 3/4 market share.
In recent years, the CFDA has approved a list of new antifungal drugs, mostly exclusive, that have been seen in the market and have not yet entered the national health care catalog. Such as MSD posaconazole oral suspension liquid (fly), Spanish Ferrer Internacional shed his health thiazole cream (ZhuoLan) and hainan Neptune left him with state pharmaceutical health thiazole cream (ling chi), hunan zhongwei pharmaceutical health thiazole of the smoothie cream, hainan sea spirit chemical pharmaceutical Lu Likang azole cream (little), Hong Kong Macao health thiazole of vision of the beauty pharmaceutical cream, vaginal tablet (Australia), chongqing winbond pharmaceutical naphthalene for Finn ketone health zun cream (bright), Shanghai modern pharmaceutical co health thiazole of bolt, etc.
varieties
Antifungal drugs are composed mainly of mimidazole, triazole, acanthopanthin, polyene, and other types of fungi. Imidazoles mainly clotrimazole, miconazole, ketone health zun, etc, these drugs tend to external use, to treat superficial fungal infections and skin mucous membrane cavity fungal infection is given priority to.
Triazole drugs mainly fluconazole and itraconazole and voriconazole, triazole drugs cytochrome P - 450 low affinity to the human body, effects of liver enzyme is lighter, can be used as a system of antifungal therapy. The acanthopterin is a new type of lipid peptide, and its representative drug is carpoffin net and micacin. Polyene antifungal drugs include photomycin B, mycostatin, and so on. Other species include terbinafine, fluorouracil, and so on.
Voliconazole: Pfizer "vivan" accounts for nearly 50 percent
Voliconazole is a triazole of pyrrolium, a product of further structural modification of fluoroconazole. The company was developed by Pfizer inc., which was first listed in the United States in 2002. Voriconazole is mainly used for invasive aspergillosis, candida of fluconazole resistance caused by severe invasive infection, pay-off bacteria genera and sickle bacteria cause of serious infection and the treatment of fungal infections in patients with immune defects, is one of the most important drug in treatment of deep fungus infection.
In 2016, Pfizer's global sales were $590 million, down 13.49% from a year earlier. In 2005, Pfizer's voliconazole was in clinical practice in China, known as "vivan". The CFDA has approved sichuan beauty huakang pharmaceutical sport, jincheng hayes pharmaceutical, chengdu Thai god health science and technology of China, the Yangtze pharmaceutical production listed eight voriconazole preparations.
According to the data from the HDM system, the amount of the drug used to be used in the public hospitals in the country was 972 million yuan in 2016, up 12.21 percent year on year. In the TOP five producers, Pfizer occupy 48.84%, sichuan mei hua kang sport occupy 13.09%, chengdu Thai god health science and technology of China occupied 11.91%, zhuhai hundred million bond occupies 9.55%, jincheng hayes occupy 6.85%.
Capifen net: the first challenge for hengrui
Mooring Finn net card for spine white fungus element class antifungal drugs, is glucan synthase inhibitors, can effectively inhibit glucan, for fungal resistance lesions has good antibacterial activity, which interfere with the fungal cell wall synthesis, is a low toxicity and efficient drugs. In 2001, the United States F.D.A. approved a net listing of capiferin, which is called Cancidas. Cancidas has a global sales of $558 million in 2016.
In 2006, carpoffin entered our country's market, known as koses. In 2016, according to HDM system data, the domestic major cities public hospital card berth Finn net booster injection drug use amount is 372 million yuan, year-on-year growth of 9.41% a year, domestic overall market size of more than 550 million yuan. On January 11, 2017, the CFDA approved the production of the kpafen pinpin, a new milestone in domestic antifungal medicine. At present, there are a lot of cap-fen papers in the review.
Fluconazole: more than 90 percent of Pfizer
Fluoroconazole is a triazole compound, which is the third antifungal drug in domestic sales. In 1990, the U.S. FDA approved the release of Pfizer's fluconazole, a product called Diflucan. The product is called "great help kang" in Chinese products. Fluconazole for broad-spectrum antifungal drug, is characterized by high bioavailability, long half-life, water-soluble, oral and intravenous drug, widely used in the treatment of superficial and deep fungal infection, the systemic candida and new cryptococcus, coccidioidomycosis, blastomycosis had a good activity and histoplasmosis.
According to the HDM system, in 2016, the amount of fluoroconazole, a key urban public hospital, was 232 million yuan, up 10.43 percent from the previous year. The injections accounted for 81.18%, the capsules accounted for 16.96 percent and tablets 1.87 percent. Of the TOP 5 companies, Pfizer accounted for 94.96 percent, 100 percent, 1.48 percent for the Yangtze, 0.65 percent for nanchang and 0.15 percent for hainan huang.
Michael: it's a big one
Mikaen is a type of acanthomycin antifungal drug developed by Japan's fujii. In March 2005, the United States FDA approved the drug, which is used to treat the treatment of esophageal candida infection, bone marrow transplant and ads-patient neutrophils reduction, and the product is called Fungusrd. In 2006 approved antifungal therapy for children, besides the yeast has good antibacterial activity, also enhanced the role of the aspergillus and so on for the treatment of invasive aspergillosis deep fungal disease such as increased new choice. In 2006, the CFDA approved the listing of the company's mikaafen injection, known as Mycamine.
According to the HDM system, in 2016, the amount of mikain, a public hospital in China's major cities, was 143m yuan, up 33% from a year earlier. The market is still dominated by anstay's mikaimin.
So, >, >, >
In recent years, the research on antifungal drugs in China and abroad has made great progress. On June 6, 2014, the FDA approved Efinaconazole, a triazole antifungal drug, from Valeant, which is used in the name Jublia, for the disease of nail fungus. On March 6, 2015, the FDA approved prior approval Japan Ann, to a new generation of triazole broad-spectrum antifungal drugs YiShaKang azole sulfate (Isavuconazonium sulfate), subject to the goods name Cresemba listed, used in the treatment of invasive aspergillosis and mucormycosis.
The development of new drugs will push for changes in the structure of antifungal products. Fungal resistance is in deep fungal infection in treatment of an increasingly serious problem, speed up the development of new antifungal drugs to deal with an increasing number of resistant fungi, is an urgent need to solve practical problems, and develop new targets for antifungal drugs will be the development direction in the future.
