| Micro-channel |
 |
|
|

|
|
|
| |
| The inflection point has now come and the vaccine market is about to enter the fast lane |
| |
| Author:中国铭铉 企划部 Release Time:2017-9-21 10:44:40 Number Browse:1946 |
| |
On September 21, 2016, as a result of the outbreak of a vaccine incident in shandong province, the vaccine market fell into freezing point, leading to a sharp decline in the performance of many vaccine companies. One year later, the vaccine industry to break the ice recovery, represented by wisdom fly biological vaccine in the first half of 2017 enterprises performance are good, plus several blockbuster vaccine products approved by the public, industry turning point is coming.
A vaccine case in shandong province has hit a low point
In March 2016, shandong police cracked an illegal vaccine case worth 570 million yuan, and the vaccine was sold to 24 provinces and cities without strict cold chain storage. Vaccines contain 25 kinds of children and adults use type 2 vaccines. After the incident, the national vaccine market fell to freezing point, the market share in the second category of vaccines shrank by more than half in 2016, and many vaccine companies were affected and the performance fell sharply.
On the policy, under the influence of "shandong illegal vaccine case", over the past two years, the government has issued multiple policies to strengthen the supervision of vaccines to avoid the recurrence of similar cases.
Promulgated by the state council, in April 2016, the state council on amending the circulation of vaccines and immunization management regulations > decision ", states: "the second type of vaccine by provincial institution of disease prevention and control organizations in provincial public resources trading platform, centralized purchasing of institution of disease prevention and control at the county level shall after purchase to vaccine production enterprises provide vaccination units in their respective administrative areas. The vaccine production enterprises shall deliver the second type of vaccine directly to the county-level prevention and control institutions, or entrust enterprises with cold chain storage and transportation conditions. Enterprises that have received the second type of vaccine entrusted to distribution shall not entrust distribution.
, in June 2016, the state administration of drug safety and national health development planning commission issued about to implement the newly revised "regulations on the administration of circulation of vaccines and vaccination, requirements are as follows:" by provincial public resources trading platform of institution of disease prevention and control purchasing vaccines, distribution enterprises shall not purchase the original vaccine vaccine, the vaccine must not be sold to outside of institution of disease prevention and control units and individuals that December 31, 2016 have purchased the second type of vaccine sales should be finished, on January 1, 2017 to stop the vaccine sales, apply for cancellation of the pharmaceutical trade license or subtract vaccine business scope."
· in September 2017, the state administration of food and drug administration and the national health and family planning commission jointly issued a notice on further strengthening the supervision and promotion of vaccine supply. The notice suggests that the vaccine should not be mixed with non-drug transport; The whole process of production, storage, transportation and use of vaccine minimum packaging units should be gradually realized. Strengthen the management of the vaccine and prevent expired vaccines from entering the use link; In the same province, autonomous region and municipality directly under the central government, the same vaccine manufacturers shall not have more than two vaccine distribution enterprises.
In the market, affected by the "shandong illegal vaccine case", the country revised the regulations on the administration of vaccine circulation and vaccination, and the service mode of vaccination has also changed. The resulting industry adjustment, adjust, adapt, make our country more than 40 about vaccine production enterprises, product sales is impacted by the large extent, led to the 2016 sales suffered significant influence, the decline of the business performance from a year earlier. Among them, Watson biology said in the 2016 half annual report that the net loss amounted to 160 million yuan. In the first half of 2016, the net profit of zhifei was 88.87%; In the second quarter of 2016, the sales of kangtai biology, due to the decline of its main business, were almost stagnant, so on January 1, 2017, it switched to direct selling mode. Tiantan biological vaccine plate in the first half of 2106 operating income of 265 million yuan, down 22.72% year on year, so transfer north 2017 research and changchun qi jian two vaccine main company equity, stripping vaccine business from now on.
The 2017 vaccine market has recovered, and the net profit of zhifei is 13 times higher
After a year, "shandong illegal vaccine case" gradually reduce the influence of the vaccine market begins to recover, through the cold winter of vaccine companies have a good harvest in the first half of 2017, to fly for typical, vaccine plate net profit was bigger than last year.
Wisdom flying creature: flying creature is a collection of vaccine research and development, production, agents, promotion, sales and distribution for the integration of biological medicine high-tech enterprises, is China's local comprehensive strength one of the strongest listed private biological vaccine supply and service providers. In the first half of 2017, the operating revenue of zhifei biology reached 445 million yuan, up 142.01 percent year-on-year. The net profit attributable to shareholders of listed companies was 172 million yuan, a year-on-year increase of 1326.96%. Wisdom fly a biological explanation results increased significantly because of shandong vaccine last year led to uncertainty factors are gradually eliminated, industry has gradually fall to the ground of the New Deal implementation, 2 kinds of vaccine sales work resumption.
Data show that wisdom flying creature existing development base 2, 1 r&d center, 21 in research projects including is applying for approval document of the project approval documents, access to clinical and preclinical, products related to epidemic encephalitis vaccine series, pneumonia vaccine, hepatitis b vaccine, tuberculosis vaccines, etc. Currently, there are 6 GMP certificates, 15 patents and 12 patent applications.
In April this year, think flying creatures come out after the first refinancing plan, proposed non-public not more than 100 million shares to raise more than $2.35 billion, flicker towards wisdom fly bamboo industrialization project of new combined vaccine (first phase), intellectual dragon horse people with rabies vaccine (diploid cells) dragon industrialization projects and intelligence division horse bio-pharmaceutical industrial park (area A) projects of research and development center, is advantageous to the company to achieve sustainable long-term development strategy.
Changchun new high: changchun gao new "freeze-dried chickenpox attenuated vaccine", "rabies vaccine" and other vaccine products have high market share in their respective fields. In the first half of 2017, the operating income of changchun is 1.6 billion yuan, up 25.44% year-on-year. Net profit attributable to listed companies was 284 million yuan, up 32.25 percent year on year. Among them, the subsidiary of the main vaccine products, the 100 grams of biological products realized revenue of 303 million yuan, an increase of 127.12 percent year on year, and realized net profit of 62.8 million yuan, up 60.58 percent year on year. In this regard, changchun high-tech explanation is due to the effect of the "shandong vaccine incident".
Biological nasal spray in changchun, the data shows, live attenuated influenza vaccine of phase III clinical complete all the volunteers, the organization held a flu Ⅲ period clinical mid-term workshop, July is expected to end of the field work of all three vaccination points in December 2017, is expected to declare the production at present. Wild dog vaccine at the same time, mai li & fung pharmaceutical products complete extension is earlier this year to 1.5 years of work, stable production process and enlarge the work achievements, the first batch of products is 1.5 years, a total of 7914 doses, in June of 2017 through batch inspection branch of the issue. All the above work has laid a foundation for meifeng's next expansion of production capacity. From January to June 2017, the vaccine application for maifeng rabies was issued by 12.13 million people, and 4.72 million people were issued by batch.
Multiple blockbuster products have been approved and the vaccine industry inflection point has been made
Previously, the domestic vaccine plate, because most of the varieties are low prices of a kind of seedling and homogenization serious type 2 seedlings, the competition is fierce, the enterprise profit is limited; Moreover, the vaccine development cycle is long, the production technology demands high, the industry barrier is higher; So it is not appreciated by the market and the enterprise. Because of the outbreak of illegal vaccines in shandong province, the vaccine market is freezing.
Last year, the CFDA approved the listing of eight vaccine products, including six domestic products and two imported products. Approved by domestic including spinal gray matter live attenuated vaccine sugar pills, people with rabies vaccine, adsorption tetanus vaccines, live attenuated varicella vaccine, freeze-dried with rabies vaccine and enterovirus type 71 inactivated vaccine; Imported products include glaxosmithkline (GSK) dual-price human papilloma virus (hirushi) and Pfizer's 13-valent pneumococcal polysaccharide (peile 13). Among them, Pfizer's global sales of pneumococcal vaccines in 2016 amounted to $57.18 billion, while the human papillomavirus vaccine in Merck 2016 was $2.2 billion in global sales. After a long winter, the vaccine market will be invigorated by the gradual recovery of the vaccine market, the proximity of more than one weight variety, such as HPV and 13 cases of pneumonia, to the initial public offerings.
Industry sources said: "the vaccine variety is the" fastest growing "innovation drug, compared with general innovative drugs, the vaccine varieties are subject to bid price reduction and medical insurance reimbursement. The terminal is a county-level CDC, and it is easy to enter the terminal process and easy to achieve sales. And with the improvement of people's living standards, residents have become more aware of the vaccine, and the vaccine has benefited from the consumption upgrade. It can be said that the large variety of vaccine is an innovative drug for upgrading, and the vast amount of space released after the launch will bring huge performance increment to the relevant enterprises, and the industrial inflection point is already present.
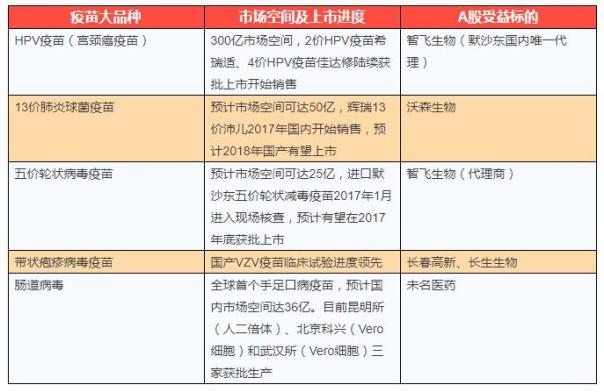
(source: zhongtai securities, changjiang securities, guangfa securities, etc.)
|
| |
Previous article:The winter has passed, five listed vaccine companies in the six months of the performance of the "big counter-attack"!
Next article:Combing 163 semi-annual reports to consider the attitude of drug companies to e-commerce
|
| |
|
|
