Medical network - March 31, priority review approval process for the drug to market opened the "door". According to the drug approval report "for 2016, a total of seven varieties except (API) approved by the public. So why is the 7 varieties to receive the discount, they can in the future in the corresponding field of "virgin" market to gain the advantage?
Reform of the implementation of the drug approval for examination and approval of height, more than a year to practice "to encourage innovation". Along with the advancement of priority review approval measures implementation, at the end of last year, a total of 193 pieces of drug registration application into priority review "fast track". On March 17, according to the state food and drug supervision and management of the administration of drug approval center released the 2016 annual drug approval report, in the examination and approval for the prior approval of registration has been completed, a total of seven varieties get advice.
1, the priority in popular approval children's drugs
According to the centre in 2016 human medicinal administration "about addressing drug registration for priority review backlog of examination and approval opinions", a total of 12 batches of 193 pieces of registration application should be brought into the priority review process.
From figure 1, you can see, in the "priority review procedures for the registration, the children use a total of 17 pieces, accounting for 9%.
Shortage of childfriendly medicine is not only caused widespread concern, but also the national government departments and medical industry needs to overcome the difficulties together. Children medicine main shortage, suitable for children of dosage forms, it is also a common problem worldwide. To solve the shortage of children's drugs, encourage a variety of our country to take measures to priority review is one of them.
In addition, in 57 a review has been completed in the application for registration of the priority review, there are 11 for proposal approved (including API registration 2 pieces).
Can be seen from table 1, complete the examination and approval and suggest a list of priority for examination and approval of registration approved, a total of seven (except for active pharmaceutical ingredients, bead) single injection has three resistance to accept order medicines, medicine with a total of four children, more than half, and the recommended three of them for import approval.
2, children's drugs - urgent clinical needs, the market space
Suggest approve the import and production of the four children's drugs are: Joe bead single injection, injection port b raschig resistance with concentrated solution, McGonagall his capsule, caffeine citrate injection, medicine for children are urgent clinical needs.
Bead an injection
Bead sheet resistance is a recombinant humanized against people interleukin 6 (IL - 6) monoclonal antibody. In March 2013, yami ROM (bead sheet resistance) approved the first indications in China with rheumatoid arthritis (RA), used in the treatment of poor response of moderately severe type activities, mainly used in adult patients. The drug, the increasing indications - type is suitable for the treatment of systemic juvenile idiopathic arthritis (sJIA), mainly used for pediatric patients, for pediatric patients in our country the first clear treatment efficacy, and safety, solves the problems of clinical medicine available so long.
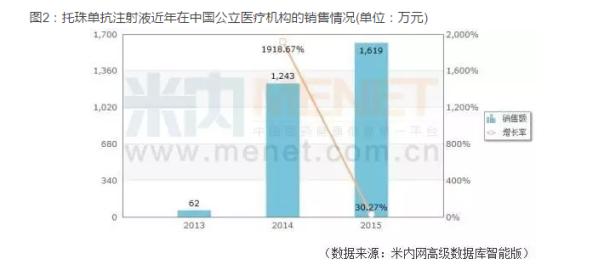
According to m Intranet database, since going public in 2013, Joe bead sheet resistance injection in public medical institutions in China (China's urban public hospitals, public hospitals at the county level, the urban community center, towns and townships) has been a steady rise in sales, in 2015 reached 16.19 million yuan. Roche said, after increasing indications, bead sheet resistance injection as China is the only approved treatment of systemic juvenile idiopathic arthritis biological agents, will have the demand of the market in 15000 children.
B raschig left temple concentrated solution for injection
Left b raschig temple is considered the "gold standard" for the treatment of epilepsy, in the United States in 1999 is the first listed in the form of tablet, was first used to partial onset seizures in adults, then its solution, injection, zyban has also listed. Recommended the import approval for Belgium optimal than pharmaceutical companies (KBC chemical) production left b raschig injection with concentrated solution, suitable for children with epilepsy.
In 2015, according to m Intranet database, left b raschig temple (tablets and solution) of public medical institutions in China sales up to 546 million yuan. At the same time, the left b raschig solution for the KBC chemical exclusive, the launch of the concentrated solution for injection in children with epilepsy huge market space.
McGonagall department he capsule
He capsule is love can tyrone McGonagall produce a glucose amide synthetase inhibitors, the product in 2006 by European regulators as orphan drug treatment of type C nyman peak disease. C NPC peak illness is the result of intracellular lipid transfer protein involved in lysosome protein genetic defects, mainly onset period for late stage infant and young, in recent years have seen a sharp rise in the number of these patients in our country. McGonagall he capsule the approved imports, for our country C nyman peak rare disease patients to provide the first effective drug treatment.
Caffeine citrate injection
Caffeine citrate injection is the only internationally approved drugs for the treatment of premature infant apnea. Premature apnea is a disabling and deadly disease, may our country always medical practice lack of effective drug treatment. And in Europe and America region, caffeine citrate preparations have become the drug of choice for clinical treatment of premature infant apnea.
Over the years, caffeine citrate injection in China's market is Italy original manufacturer alpha WeiShiMan pharmaceutical company exclusive occupation. , according to m Intranet database in public medical institutions in China in 2015 sales of 34.46 million yuan. The chengdu YuanDong pharmaceutical co., LTD., approved by the production of caffeine citrate injection for domestic first imitation drugs, is expected to reduce the patients' medical costs.
The treatment 3, -- the first copy is broken billion market
Is the treatment for, targeting advanced non-small cell lung cancer with epidermal growth factor receptor's first generation of small molecule tyrosine kinase inhibitors, compared with the traditional chemotherapy efficacy and safety were better, and exclusive production developed by astrazeneca.
, according to senior m Intranet database in small molecules targeting antineoplastic drugs used in treatment of lung cancer, the treatment produced by astrazeneca in recent years in sales and market share in the first place, the public medical institutions in China in 2015 sales of 1.253 billion yuan.
The treatment of the qilu pharmaceutical (r) brand name: Iraq as our country the first drug approved listing, will effectively improve the accessibility of drugs in patients with. At the same time, according to introducing, qilu pharmaceutical Iraq, but on the premise of consistent with the original drug quality, lower price after the price of the original drug by 1/3, and astrazeneca broken more than ten of the market.
4, rui GeFei piece - a "alone" to gain the market
Rui GeFei for small molecule tyrosine kinase inhibitors, developed jointly by bayer and Onyx. Through the examination and approval procedures for priority review of rui GeFei, approved the import and is suitable for the treatment of always received with fluorouracil, and oxaliplatin into Iraq for kang based chemotherapy, and always received or not suitable for accepting resistance against vascular endothelial growth factor receptor, epidermal growth factor receptor drugs in the treatment of wild type (RAS) of patients with metastatic colorectal cancer; Always received imatinib mesylate and malic acid chougny for treatment of locally advanced and unable to surgical resection or metastatic gastrointestinal stromal tumor patients.
Rui GeFei relevant data show that 2015 sales of $347 million, up 17% from a year earlier. The because of its "significant clinical advantage" in China approved by the public, the drug for our country the first used in the treatment of advanced colorectal cancer molecular targeted drugs, is expected to be over a period of time with the advantages of a "alone" occupy the market.
However, rui GeFei, how long can slice in the Chinese market "thrive" worthy of attention. It is reported that there have been many domestic enterprises in accordance with 3.1 class new medicine to apply for registration, the future r GeFei, can be very competitive in the domestic market.
5, mooring Finn net card - acetic acid for injection patents expire beat machine
Mooring Finn net card original manufacturer for Merck, suitable for adults and children patients (three months and more than three months) empirical treatment of neutropenia, associated with fever patients of suspected fungal infection and the treatment of other invalid or intolerance to invasive aspergillus. Acetic acid card berth Finn were developed by Merck & Co (Merck) listed a semisynthetic beta for intravenous - 1, 3 - producing glucan synthesis inhibitors, is the first listed the spines of white fungus element class antifungal agent.
Mooring Finn net card, according to m Intranet database, 2015 sales of public medical institutions in China has more than 1 billion yuan, and after the market has been the original manufacturers MSD sole possession. The hengrui pharmaceutical launched the first imitation drugs seized the opportunity of patents expire, through prior approval procedures approved by the public, also is the present domestic only one. Hengrui medicine, said the move helped popularize the products in the domestic market, mooring Finn net card is expected to enter the rapid growth, became the company's big new varieties.
conclusion
As one of the "punch" human medicinal reform, priority review for examination and approval procedures for solving patients is not meet the clinical needs, and promote the development of pharmaceutical industry in China, has important significance. Drug firms also benefit from the "fast track", let advantage varieties are listed, rapid production of these products in the future can play power in the market, we hope. |
